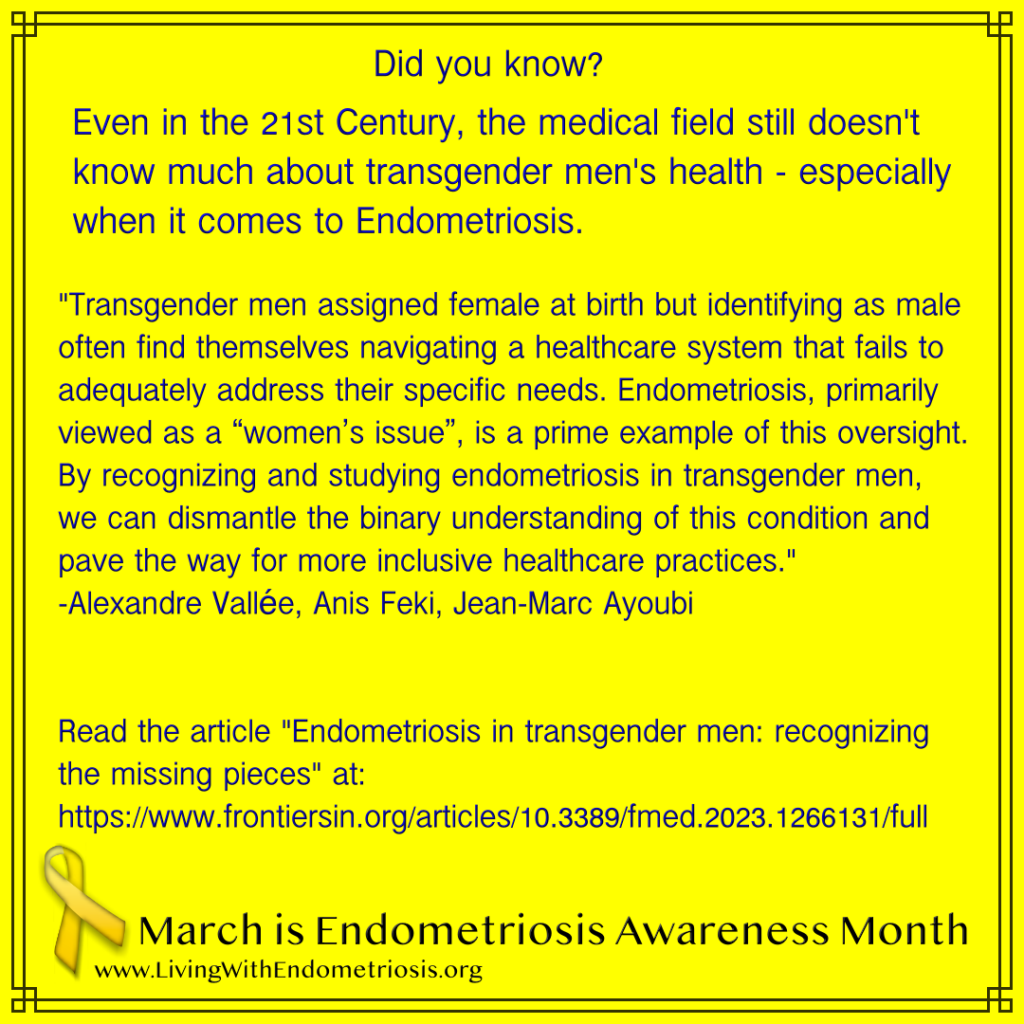
On this final day of Endometriosis Awareness Month, it is also Transgender Day of Visibility, and I stand firmly with my Trans Endometriosis Siblings in solidarity as we strive for changes toward equitable treatment in worldwide health care.

Image courtesy endoqueer.com.